Enterokinase Cleavage Enzyme for Protein Purification from ABM, 100 units
Description The Enterokinase included in this kit is the catalytic
subunit of the native holoenzyme, and is highly active and specific for cleaving fusion proteins with the recognition sequence, DDDDK, in the interdomain linker.
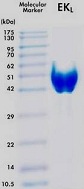
Because this product is produced from mammalian expression system, it is highly glycosylated and shows extremely specific cleavage activity compared to other E. coli produced Enterokinases. The purified Enterokinase behaves as a 47kD band under denaturing and reducing conditions as visualized on SDS-PAGE.
Applications Enterokinase is a site-specific protease that recognizes and cleaves after the C-terminal end of lysine residue in the recognition sequence, DDDDK. Unlike other site-specific proteases that cut within the recognition sequences leaving extra amino acids in the cleaved peptide products, the C-terminal peptide fragment produced from the Enterokinase cleavage reaction doesn't inherent any residues from the DDDDK recognition sequence*. Therefore, the application can be extremely advantageous for producing a 100% native protein sequence and structure from recombinant fusion protein,
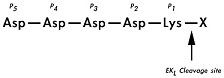
which has the desired product immediately after the Enterokinase recognition sequence, DDDDK. [*Note: Enterokinase will not cleave at site when lysine is followed by proline.]
In addition, DDDDK is part of the octapeptide FLAG tag (DYKDDDDK), which has been used as a fusion tag for recognition by antibody and detection of fusion protein expression with Western blot analysis, and for purification of the fusion protein by Anti-FLAG affinity chromatography. This array of applications makes Enterokinase an ideal tool in the research involving the study of protein structure and function, and protein production where native protein structures and sequences are desired.
Components Enterokinase: 100 units in 100ul
***One unit of Enterokinase will cleave 50µg of cleavage control protein to 95% completion in 16hrs at 25°C.***
Special Features
No residues left from recognition sequence after cleavage. Produced in mammalian expression system. Allows removal of tags after use in purification. Excellent price. References
Baratti, J., Maroux, S., and Louvard, D. (1973). Effect of Ionic Strength and Calcium Ions on the Activation of Trypsinogen by Enterokinase. A Modified Test for the Quantitative Evaluation of the Enzyme. Biochem. Biophys. Acta 321: 632-638. Choi, S.I., Song, H.W., Moon, J.W., and Seong, B.L. (2001). Recombinant Enterokinase Light Chain with Affinity Tag: Expression from Saccharomyces cerevisiae and Its Utilities in Fusion Protein Technology. Biotechnol. Bioeng. 75: 718-724. LaVallie, E.R., Rehemtulla, A., Racie, L.A., Diblasio, E.A., Ferenz, C., Grant, K.L., Light, A., and McCoy, J.M. (1993). Cloning and Functional Expression of a cDNA Encoding the Catalytic Subunit of Bovine Enterokinase. J. Biol. Chem. 268: 23311-23317. Lu, D., Fűtterer, K., Korolev, S., Zheng, X., Tan, K., Waksman, G., and Sadler, J.E. (1999) Crystal Structure of Enteropeptidase Light Chain Complexed with an Analog of the Trypsinogen Activation Peptide. J. Mol. Biol. 292: 361-373. Maroux, S., Baratti, J., and Desnuelle, P. (1971). Purification and Specificity of Porcine Enterokinase. J. Biol. Chem. 246: 5031-5039.